

This is one of many pages about types of organic compounds in our Organic Chemistry Section. Some of this information is also useful for students of GCSE Chemistry.
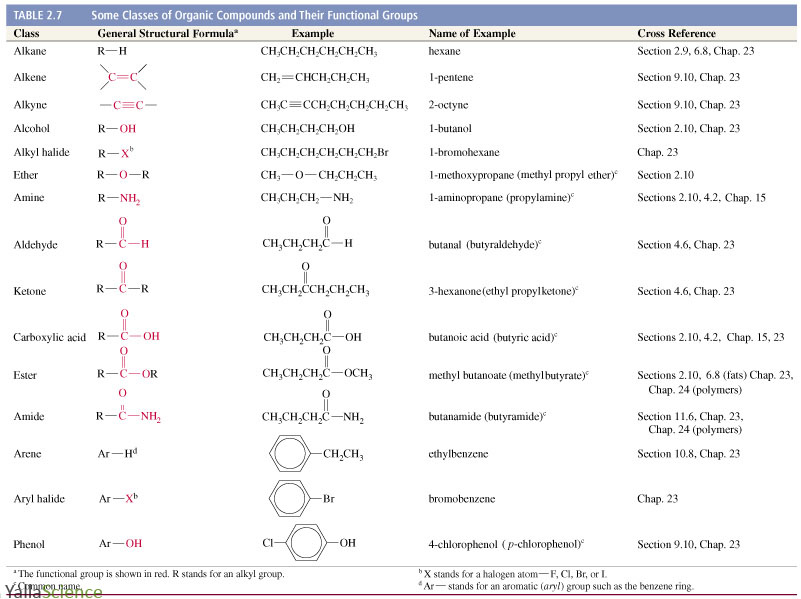
The above table of organic chemistry functional groups is not a complete list but includes those functional groups required by most UK A-Level Chemistry (that is, AS Chemistry and A2 Chemistry combined) exam boards. Review the table above then visit the links to further examples in the first column (left-side) of the table. When studying the structures and naming of organic molecules it is useful to see and compare many examples of similar chemicals and their structures. For example, see the structures of propanal and propanone (above). This is due to the existence of multiple isomers of some combinations of elements. However, although this is a useful way to identify any wrong (impossible!) molecular structures, this check alone is not sufficient to guarantee that a structure is drawn correctly. Therefore when double bonds are represented by two parallel lines and triple bonds are represented by three parallel lines it is easy to check if molecular structures are at least possible by counting the total number of straight lines extending from each atom.
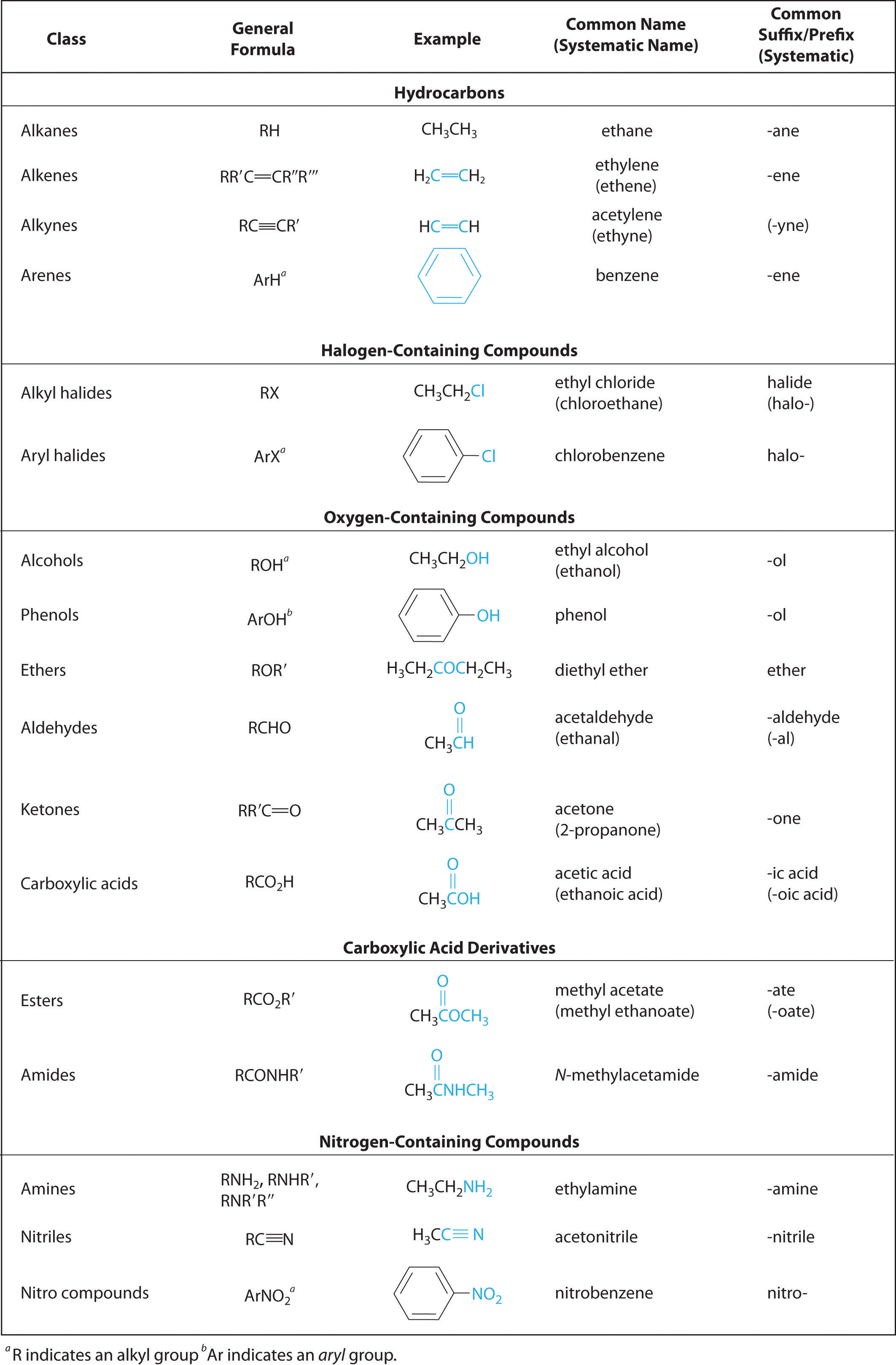
The purpose of the detailed representation here is to show how easy it is to check that you are drawing the correct structure(s) by counting the lines (bonds) extending away from each type of atom. Many chemistry textbooks, websites and other sources show simpler representations of large or complex molecules in order to save space and / or for clarity. That is, every chemical bond is represented by a line (or lines, in the cases of double and triple bonds). The chemical structures shown in the right-hand column above have been drawn out in full.
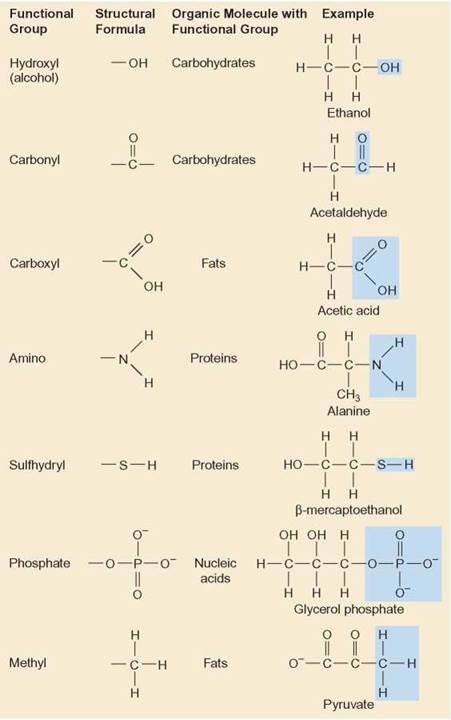
See the links indicated for more examples of chemicals that include the functional groups featured here. The prefix is used in some circumstances and the suffix is used in other circumstances so it is useful to be aware of both.Įxamples: Simple examples (of chemicals with 3 carbon atoms forming a chain, and hence whose molecules are relatively small) have been chosen as examples in order to fit diagrams of the structures of all molecules into a single table relatively easily. Naming: As is clear from the examples, when functional groups are associated with both a prefix and a suffix these are not used at one (in the same chemical name).


 0 kommentar(er)
0 kommentar(er)
